Leucine and Muscle Protein Synthesis
One of the few nutritional supplements that an individual participated in exercise and sports at any level in his/her life has probably heard of is “branched chain amino acids”, otherwise known as BCAA. Protein structures in our body are formed by the combination of 20 different amino acids. Of these 20 amino acids, 9 are classified as essential amino acids, that is, they cannot be synthesized by the body and must be taken from the outside through food. At this stage, it is necessary to open a separate parenthesis for arginine and histidine amino acids. These amino acids can be classified as essential amino acids according to the physiological period and situation (growth and development age, pregnancy, trauma, etc.), which is called “conditionally essential amino acid”. Leucine, isoleucine and valine amino acids, which are included in these 9 essential amino acids, form the branched chain amino acids group, which we will talk about in our article and examine their relationship with muscle protein synthesis.
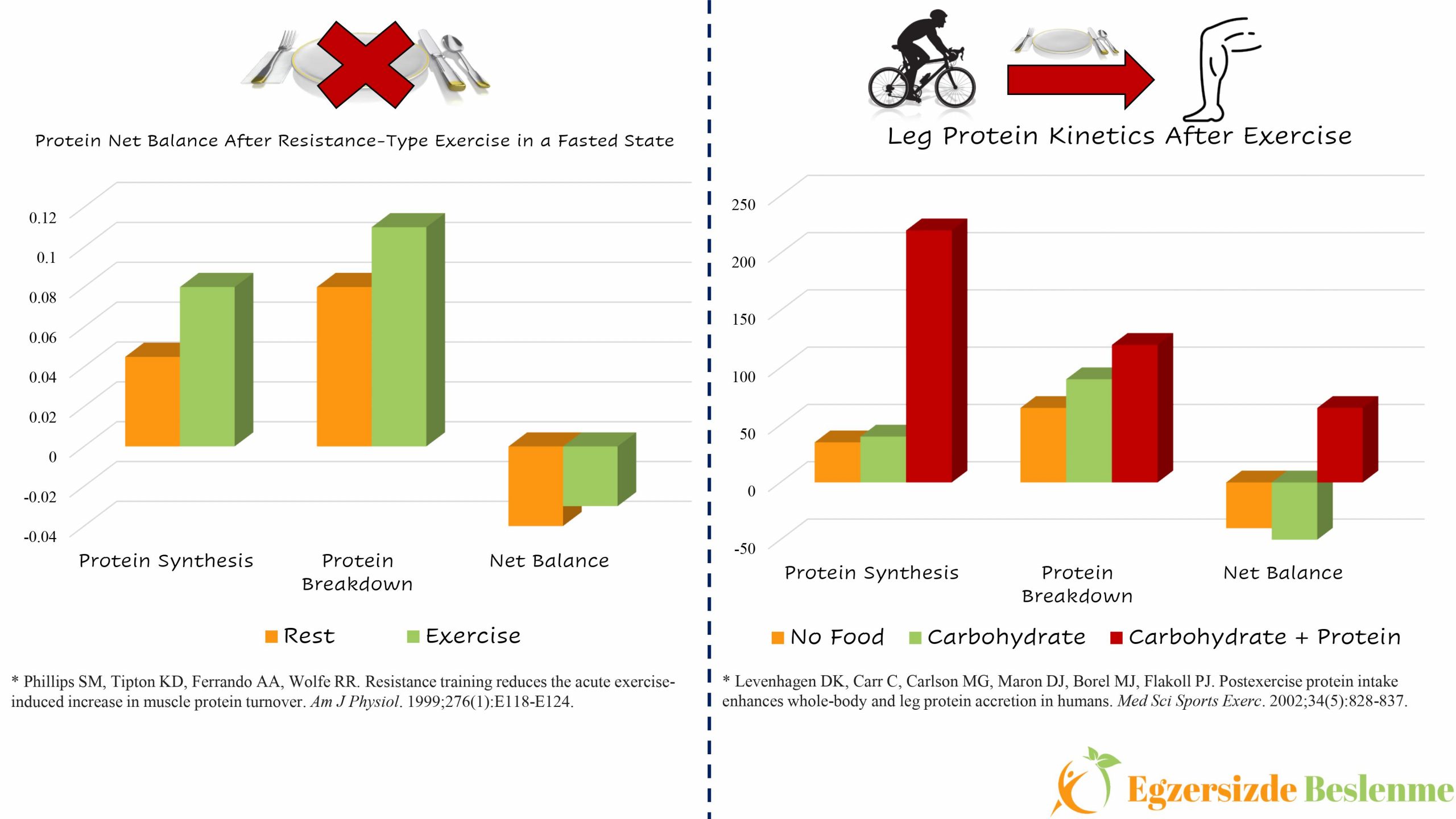
Muscle protein synthesis occurs when the rate of muscle protein synthesis exceeds the rate of degradation. If we first examine the relationship between muscle protein synthesis and exercise, we know that muscle protein synthesis decreases during exercise compared to resting state, on the contrary it increases after exercise (1). While muscle protein breakdown does not change or decreases according to the resting state during exercise (more research is still needed on this subject), it definitely increases after exercise (2). According to this information, when we exclude the nutrition factor from the equation, the picture reveals that muscle protein balance value will remain in the negative part as muscle protein breakdown will be higher compared to its synthesis following exercise performed in the fasted state (3).
So, when we add the nutrition factor into this equation, what kind of picture do we see? Seeking an answer to this very question, Levenhagen and his study group had a team of 10 volunteers consisting of 5 men and 5 women who performed 60 minutes of cycling training at 60% of the maximal oxygen consumption (VO2max) with 3 different nutrition protocols (4). These 3 different nutrition protocols are as follows; a) no food was consumed, b) a meal containing 8 g carbohydrate, 3 g fat, c) a meal containing 10 g protein, 8 g carbohydrate and 3 g fat was consumed. The effects of these meals, which are consumed immediately after exercise, on the protein synthesis and breakdown kinetics in the legs were investigated. While the net protein balance remained in the negative area similarly in the “a” and “b” protocols, the net protein balance value after the c protocol was in the positive area. The result of this study shows that in order to provide positive gain in terms of muscle protein after exercise, protein must be included in the meal consumed after exercise.
You may ask that “When are we going to include branched chain amino acids in this equation?” Luckily, another scientist who asked this question and his study group conducted a study where a group of 8 volunteers, while performing 60 minutes of cycling exercise at 60% VO2peak difficulty, and applied 2 different nutritional supplement protocols on the bike (5). The difference between the applied nutritional supplementation protocols is that one of the 10 g essential amino acid mixtures contains 1.87 g leucine, while the other essential amino acid mixture contains 3.5 g of leucine. The results of this study showed that the mixture of essential amino acids with higher leucine content resulted in 33% higher muscle protein synthesis in “vastus lateralis” compared to the other mixture.
However, by approaching this study with a critical eye; “Is there a limit to the power of leucine-led branched-chain amino acids to increase muscle protein synthesis?”, “Does a mixture with the same amount of leucine but a lack of other essential amino acids still have the same power to increase muscle protein synthesis?” Answering these questions will provide us with a clearer framework. Let’s imagine that we created a study protocol to answer these questions. In this study, our volunteers perform a resistance exercise, that will stimulate muscle protein synthesis, by applying 2 different nutritional supplementation protocols. The difference between these nutritional supplementation protocols is that although they contain the same amount of leucine amino acid, one of the mixtures contains insufficient essential amino acids, while the other contains sufficient (such as, 6.25 grams of Whey protein mixture containing 3 g of leucine and 25 grams of Whey protein containing 3 g of leucine). In order to reveal the effect of these nutritional supplementation protocols on muscle protein synthesis in “vastus lateralis”, muscle biopsies should be taken from this region immediately after resistance exercise, at the end of the 1st hour, at the end of the 3rd hour and at the end of the 5th hour and these values should be compared. As a result of this study, we would have obtained the answer to one of the questions we asked above. Churchward-Venne and the working group carried out a similar (actually much better) study of which we proposed. As a result of this study, although the leucine content is equal, the Whey protein mixture, which is insufficient in terms of other essential amino acids, was able to go head-to-head with the Whey protein mixture containing other essential amino acids in sufficient amount in terms of “vastus lateralis” muscle protein synthesis in the first 1 to 3-hour period. However, between the 3rd and 5th hours the level of protein synthesis was insufficient (6).
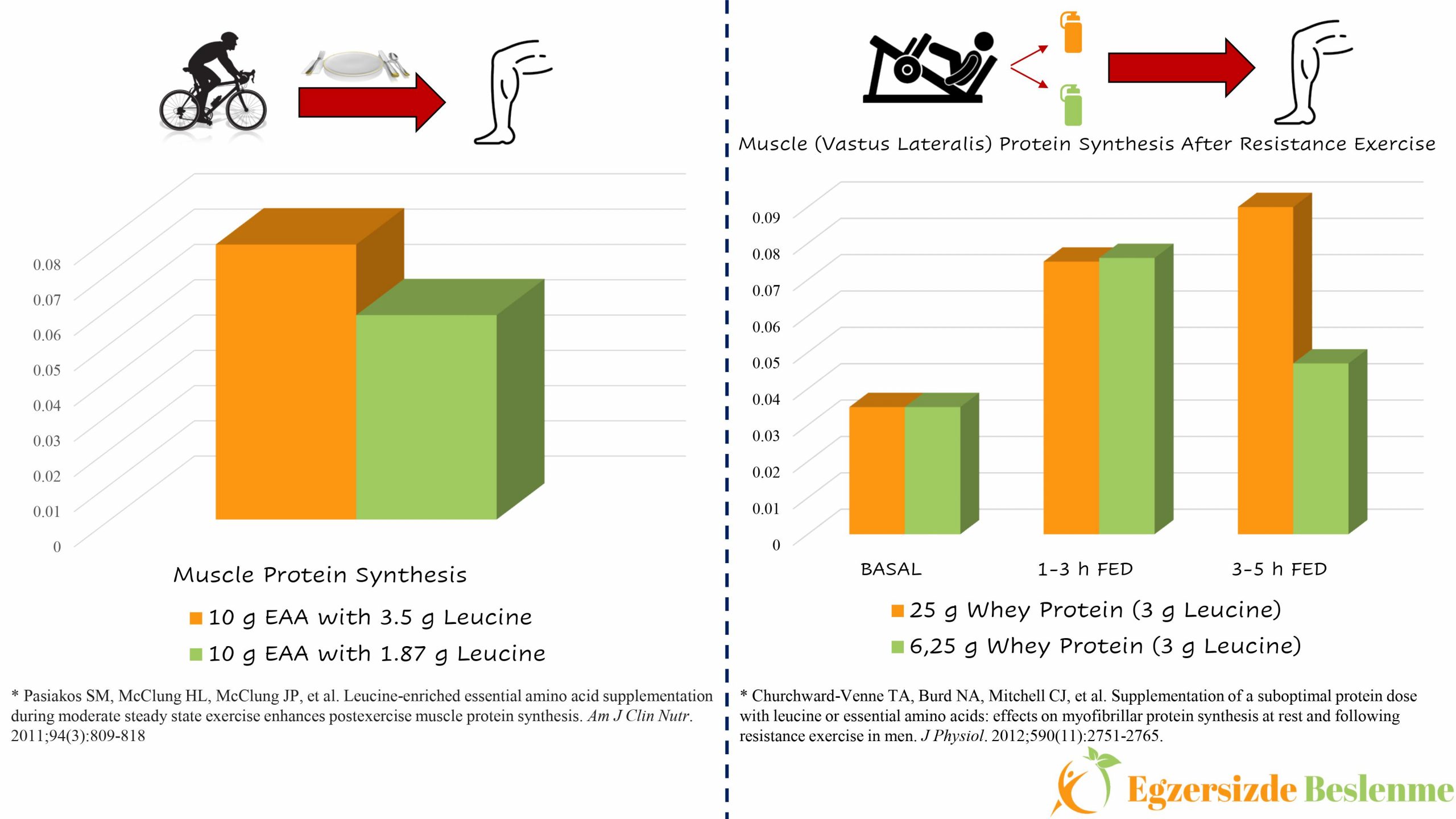
In the light of the data obtained from the above-mentioned studies and other studies carried out in this field, the most cautious interpretation that can be made is; Nutritional supplements containing branched-chain amino acids or nutritional supplements containing only the amino acid leucine itself in the absence of other essential amino acids can increase muscle protein synthesis and suppress muscle protein breakdown immediately after consumption, however, they may be insufficient to increase muscle protein synthesis and/or decrease protein breakdown in the hours following exercise compared to protein mixtures containing sufficient amounts of all essential amino acids(7,8).
References
- Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576(Pt 2):613-624.
- Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol (1985). 2009;106(6):2026-2039.
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276(1):E118-E124.
- Levenhagen DK, Carr C, Carlson MG, Maron DJ, Borel MJ, Flakoll PJ. Postexercise protein intake enhances whole-body and leg protein accretion in humans. Med Sci Sports Exerc. 2002;34(5):828-837.
- Pasiakos SM, McClung HL, McClung JP, et al. Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr. 2011;94(3):809-818.
- Churchward-Venne TA, Burd NA, Mitchell CJ, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol. 2012;590(11):2751-2765.
- Wolfe RR. Branched-chain amino acids and muscle protein synthesis in humans: myth or reality?. J Int Soc Sports Nutr. 2017;14:30.
- Jäger R, Kerksick CM, Campbell BI, et al. International Society of Sports Nutrition Position Stand: protein and exercise. J Int Soc Sports Nutr. 2017;14:20.
